Pde4 inhibitor copd
Home » » Pde4 inhibitor copdYour Pde4 inhibitor copd images are available. Pde4 inhibitor copd are a topic that is being searched for and liked by netizens now. You can Get the Pde4 inhibitor copd files here. Find and Download all free images.
If you’re searching for pde4 inhibitor copd images information related to the pde4 inhibitor copd keyword, you have come to the right blog. Our website always gives you suggestions for refferencing the highest quality video and image content, please kindly search and find more enlightening video content and images that fit your interests.
Pde4 Inhibitor Copd. Roflumilast-n-oxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an add-on to standard treatment. It is swallowed by mouth in a pill that is taken once a day every day. Gastrointestinal adverse effects and weight loss were common and safety data submitte. PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models.
 Mechanism Of Action Of Phosphodiesterase 4 Inhibitors Pdeis Pde4is Download Scientific Diagram From researchgate.net
Mechanism Of Action Of Phosphodiesterase 4 Inhibitors Pdeis Pde4is Download Scientific Diagram From researchgate.net
Phosphodiesterase-4 PDE₄ inhibitors are a relatively new class of medicines marketed to improve COPD. Indeed both the FDA and EMEA approved roflumilastnoxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an addon to standard treatment. The treatment of COPD is the most studied utility for PDE4 inhibitors due to their ability to inhibit inflammatory cell responses. Roflumilast-n-oxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an add-on to standard treatment. Outcomes With Roflumilast a PDE4 Inhibitor for the Treatment of COPD Dennis Williams PharmD BCPS AE-C12 Abstract Purpose. Two currently available medicines - roflumilast and cilomilast - are taken as a tablet.
FDA-approved to reduce the risk of chronic obstructive pulmonary disease COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations.
Hence these agents represent a therapeutic advance in the treatment of COPD due to their novel mechanism of action and potent anti-inflammatory effects coupled with a good safety and tolerability profile. To date inflammatory cells 12. No PDE4 inhibitors have been approved for clinical use. Orally administered PDE4 inhibitors do however have a low thera-. TRANSLATIONAL AND MECHANISTIC STUDIES Efficacy and Safety of CHF6001 A Novel Inhaled PDE4 Inhibitor in COPD. Phosphodiesterase-4 PDE₄ inhibitors are a relatively new class of medicines marketed to improve COPD.
 Source: researchgate.net
Source: researchgate.net
Efficacy and Safety of CHF6001 A Novel Inhaled PDE4 Inhibitor in COPD. The Pioneer Dose Finding Study. FDA-approved to reduce the risk of chronic obstructive pulmonary disease COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. Gastrointestinal adverse effects and weight loss were common and safety data submitte. Phosphodiesterase-4 PDE₄ inhibitors are a relatively new class of medicines marketed to improve COPD.
 Source: researchgate.net
Source: researchgate.net
Roflumilast is a targeted oral once-daily administered phosphodiesterase 4 PDE4 inhibitor with clinical efficacy in chronic obstructive pulmonary disease COPD. PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models. The only PDE4 inhibitor that is currently approved to treat COPD is called Daliresp roflumilast. Efficacy and Safety of CHF6001 A Novel Inhaled PDE4 Inhibitor in COPD. No PDE4 inhibitors have been approved for clinical use.
 Source: researchgate.net
Source: researchgate.net
Indeed both the FDA and EMEA approved roflumilastnoxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an addon to standard treatment. Phosphodiesterase 4 PDE4 is a major cyclic adenosine-35-monophosphate-metabolizing enzyme in immune and inflammatory cells airway smooth muscle and pulmonary nerves. Selective inhibitors of this enzyme have been available for a number of years and show a broad spectrum of activity in animal models of COPD and asthma. Roflumilast is a targeted oral once-daily administered phosphodiesterase 4 PDE4 inhibitor with clinical efficacy in chronic obstructive pulmonary disease COPD. In people with COPD PDE 4 inhibitors offered benefit over placebo in improving lung function and reducing the likelihood of exacerbations.
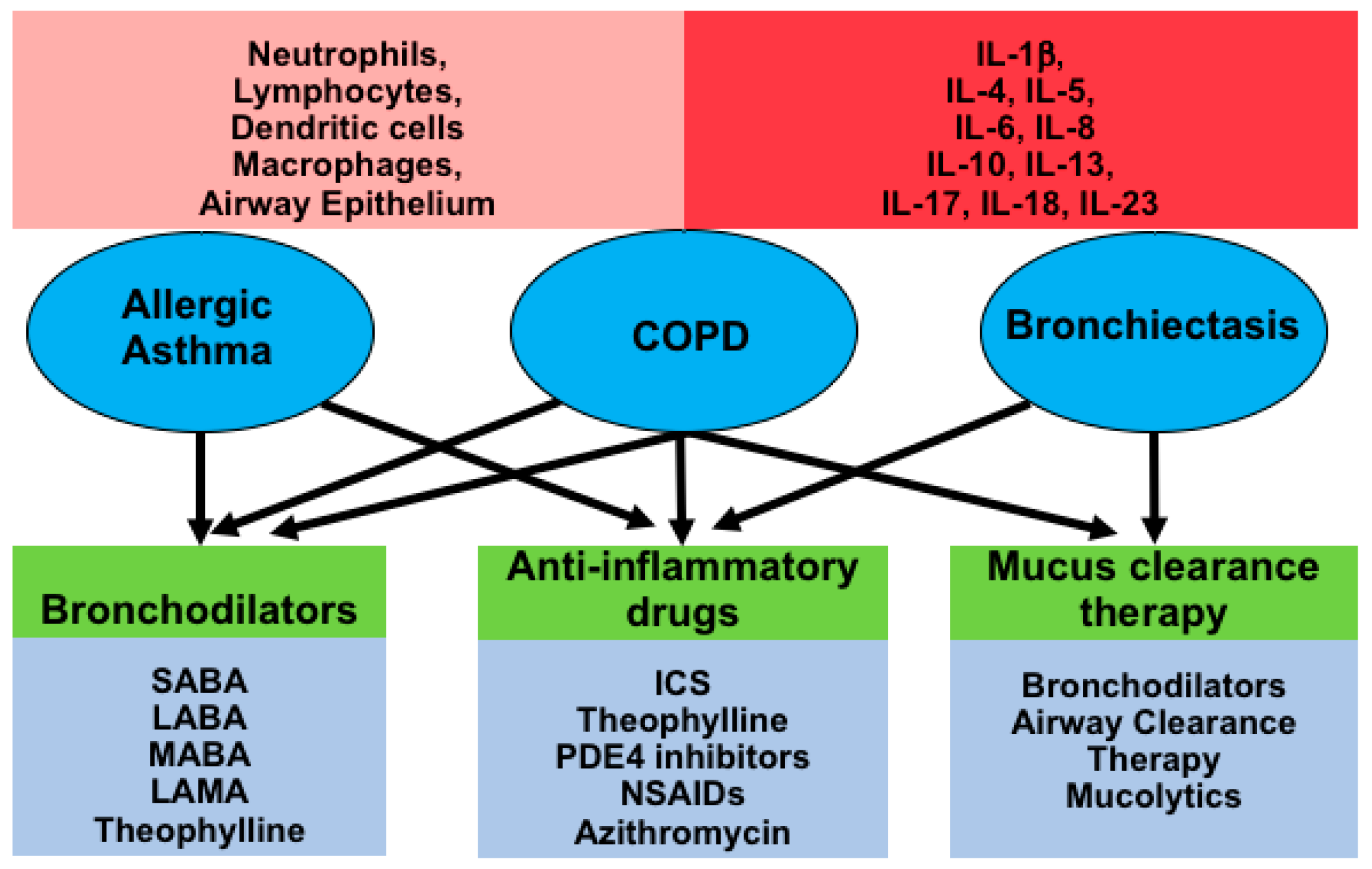 Source: mdpi.com
Source: mdpi.com
Phosphodiesterase 4 PDE4 is a major cyclic adenosine-35-monophosphate-metabolizing enzyme in immune and inflammatory cells airway smooth muscle and pulmonary nerves. They have both bronchodilator and anti-inflammatory effects. In contrast the selective PDE4 inhibitors are convenient medications for both patient and physician alike. The pharmacology of roflumilast recent dosing revisions and the integral roles of pharmacists in effective chronic obstructive pulmonary disease COPD management are. PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models.
 Source: jacionline.org
Source: jacionline.org
The Pioneer Dose Finding Study. Selective inhibitors of this enzyme have been available for a number of years and show a broad spectrum of activity in animal models of COPD and asthma. PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models. FDA-approved to reduce the risk of chronic obstructive pulmonary disease COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. However cilomilast Ariflo 39 has received an approvable letter from the US Food and Drugs Administration.
 Source: researchgate.net
Source: researchgate.net
PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models. It is swallowed by mouth in a pill that is taken once a day every day. Phase I increases in intracellular cAMP which in turn results in studies in healthy volunteers have indicated that once daily smooth muscle relaxation and suppression of activation of dosing with 5 mg of BAY 19-8004 is optimal. The agent is not to be used as a bronchodilator and is not indicated for the relief of acute bronchospasm. The orally administered PDE4 inhibitor roflumilast prevents exacerbations in patients with COPD although is effective only in a specific subgroup.
 Source: sciencedirect.com
Source: sciencedirect.com
The orally administered PDE4 inhibitor roflumilast prevents exacerbations in patients with COPD although is effective only in a specific subgroup. Roflumilast-n-oxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an add-on to standard treatment. The Pioneer Dose Finding Study. Inhibition of PDE4 results in treatment of patients with asthma or COPD. Hence these agents represent a therapeutic advance in the treatment of COPD due to their novel mechanism of action and potent anti-inflammatory effects coupled with a good safety and tolerability profile.
 Source: thelancet.com
Source: thelancet.com
PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models. Phosphodiesterase-4 PDE4 inhibition has an established role in the management of chronic obstructive pulmonary disease COPD. The agent is not to be used as a bronchodilator and is not indicated for the relief of acute bronchospasm. The only PDE4 inhibitor that is currently approved to treat COPD is called Daliresp roflumilast. Gastrointestinal adverse effects and weight loss were common and safety data submitte.
 Source: researchgate.net
Source: researchgate.net
The orally administered PDE4 inhibitor roflumilast prevents exacerbations in patients with COPD although is effective only in a specific subgroup. Results from in vitro studies with roflumilast indicate that it has anti-inflammatory properties that may be applicable for the treatment of COPD. Gastrointestinal adverse effects and weight loss were common and safety data submitte. They have both bronchodilator and anti-inflammatory effects. To date inflammatory cells 12.
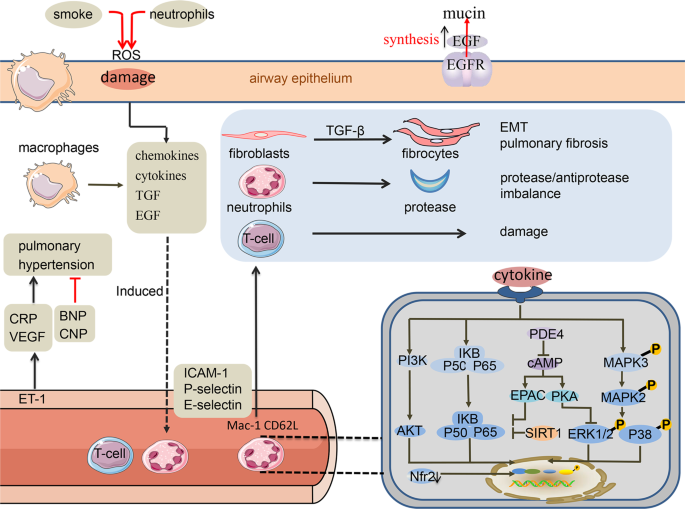 Source: nature.com
Source: nature.com
Results from in vitro studies with roflumilast indicate that it has anti-inflammatory properties that may be applicable for the treatment of COPD. Gastrointestinal adverse effects and weight loss were common and safety data submitte. Email to a Friend. Outcomes With Roflumilast a PDE4 Inhibitor for the Treatment of COPD Dennis Williams PharmD BCPS AE-C12 Abstract Purpose. However systemic exposure after oral administration can cause side effects such as nausea weight loss and gastrointestinal disturbance which may limit its use in clinical practice.
 Source: researchgate.net
Source: researchgate.net
They have both bronchodilator and anti-inflammatory effects. The Pioneer Dose Finding Study Abstract Send to Citation Mgr. Indeed both the FDA and EMEA approved roflumilastnoxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an addon to standard treatment. The orally administered PDE4 inhibitor roflumilast prevents exacerbations in patients with COPD although is effective only in a specific subgroup. Patients are usually prescribed a bronchodilator to use in combination with Daliresp.
 Source: bcrt.ca
Source: bcrt.ca
Phosphodiesterase 4 PDE4 is a major cyclic adenosine-35-monophosphate-metabolizing enzyme in immune and inflammatory cells airway smooth muscle and pulmonary nerves. FDA-approved to reduce the risk of chronic obstructive pulmonary disease COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations. The pharmacology of roflumilast recent dosing revisions and the integral roles of pharmacists in effective chronic obstructive pulmonary disease COPD management are. However they had little impact on quality of life or symptoms. Two currently available medicines - roflumilast and cilomilast - are taken as a tablet.
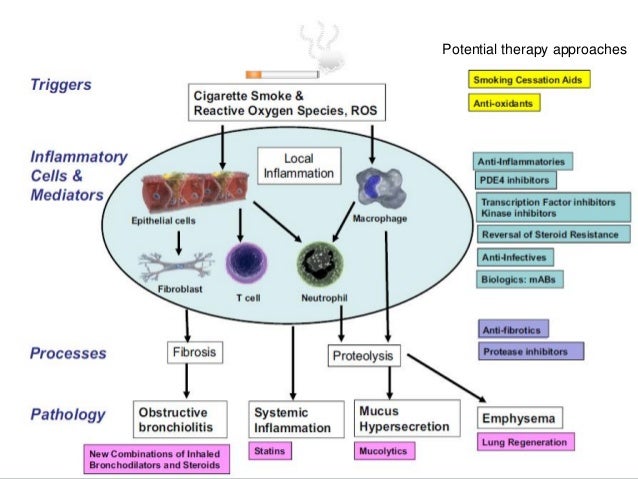 Source: slideshare.net
Source: slideshare.net
However systemic exposure after oral administration can cause side effects such as nausea weight loss and gastrointestinal disturbance which may limit its use in clinical practice. PDE4 inhibitors have been widely investigated for the treatment of asthma and COPD because of their well-established anti-inflammatory activity in a variety of cellular and animal models. Two currently available medicines - roflumilast and cilomilast - are taken as a tablet. The Pioneer Dose Finding Study Abstract Send to Citation Mgr. Phosphodiesterase-4 PDE₄ inhibitors are a relatively new class of medicines marketed to improve COPD.
 Source: researchgate.net
Source: researchgate.net
Indeed both the FDA and EMEA approved roflumilastnoxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an addon to standard treatment. Roflumilast is a targeted oral once-daily administered phosphodiesterase 4 PDE4 inhibitor with clinical efficacy in chronic obstructive pulmonary disease COPD. No PDE4 inhibitors have been approved for clinical use. The PDE4 enzyme has been proven to be a versatile drug target for therapeutics to treat diverse disease conditions such as asthma COPD diabetes Huntingtons disease and various other inflammatory disorders. It is swallowed by mouth in a pill that is taken once a day every day.
 Source: cell.com
Source: cell.com
It is swallowed by mouth in a pill that is taken once a day every day. In people with COPD PDE 4 inhibitors offered benefit over placebo in improving lung function and reducing the likelihood of exacerbations. Selective inhibitors of this enzyme have been available for a number of years and show a broad spectrum of activity in animal models of COPD and asthma. No PDE4 inhibitors have been approved for clinical use. Email to a Friend.
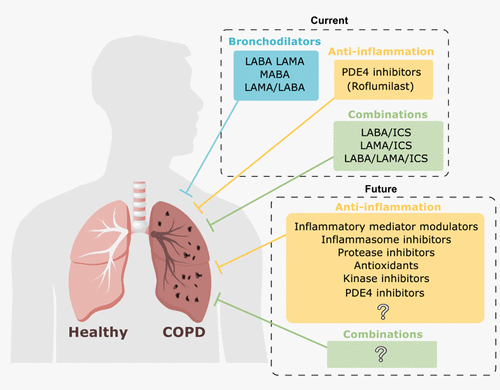 Source: x-mol.com
Source: x-mol.com
However they had little impact on quality of life or symptoms. Phosphodiesterase-4 PDE₄ inhibitors are a relatively new class of medicines marketed to improve COPD. The Pioneer Dose Finding Study Abstract Send to Citation Mgr. Hence these agents represent a therapeutic advance in the treatment of COPD due to their novel mechanism of action and potent anti-inflammatory effects coupled with a good safety and tolerability profile. TRANSLATIONAL AND MECHANISTIC STUDIES Efficacy and Safety of CHF6001 A Novel Inhaled PDE4 Inhibitor in COPD.
 Source: researchgate.net
Source: researchgate.net
Indeed both the FDA and EMEA approved roflumilastnoxide an orally administered selective PDE4 inhibitor in 2010 for the maintenance treatment of severe COPD associated with bronchitis and a history of frequent exacerbations as an addon to standard treatment. The treatment of COPD is the most studied utility for PDE4 inhibitors due to their ability to inhibit inflammatory cell responses. Inhibition of PDE4 results in treatment of patients with asthma or COPD. Patients are usually prescribed a bronchodilator to use in combination with Daliresp. Two currently available medicines - roflumilast and cilomilast - are taken as a tablet.
 Source: semanticscholar.org
Source: semanticscholar.org
The treatment of COPD is the most studied utility for PDE4 inhibitors due to their ability to inhibit inflammatory cell responses. Phase I increases in intracellular cAMP which in turn results in studies in healthy volunteers have indicated that once daily smooth muscle relaxation and suppression of activation of dosing with 5 mg of BAY 19-8004 is optimal. TRANSLATIONAL AND MECHANISTIC STUDIES Efficacy and Safety of CHF6001 A Novel Inhaled PDE4 Inhibitor in COPD. Individuals with chronic bronchitis and a history of exacerbations 3456. No PDE4 inhibitors have been approved for clinical use.
This site is an open community for users to do submittion their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title pde4 inhibitor copd by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.